What is the Law of Conservation of Matter? (Worksheets and Explanation)
Grade 8 Science Worksheets
The law of conservation of matter, also known as the law of conservation of mass, states that in a closed system, matter cannot be created or destroyed; it can only change forms or be rearranged. The total mass of the substances involved in a chemical reaction remains constant before and after the reaction.
Click here to jump straight to the Law of Conservation of Matter.
Molecules and Matter
The Universe is made of Matter. All matter occupies space. Mass is the amount of matter in an object. It is the sum of all the Molecules which make up that matter. Molecules are packed differently in different matter.
What is the Law of Conservation of Matter?
The Law of Conservation of Matter states that matter can neither be created nor destroyed. Matter can change form through physical or chemical changes but in both cases, the matter is conserved.
Take the example of a physical change – water freezes to form ice or ice melts to form water. In both these changes, the number of molecules of water remains exactly the same before and after the physical change. In other words, the matter is neither created nor destroyed.
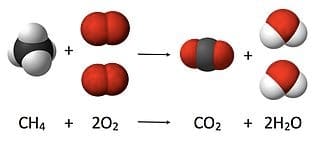
Now let’s look at a chemical change – hydrogen and oxygen atoms react chemically to form water. It is represented by a balanced chemical equation as below:
2H2 (g) + O2 (g) → 2H2O (l)
The equation shows that 4 atoms of hydrogen or 2 molecules of hydrogen gas react with 2 atoms of oxygen or one molecule of oxygen gas, resulting in 2 molecules of a liquid named water, each molecule containing 2 atoms of hydrogen and one atom of oxygen. In other words, the total number of hydrogen and oxygen atoms (4 and 2 respectively) remains exactly the same before and after the chemical reaction.
The same number of atoms of hydrogen and oxygen before a chemical reaction gets arranged differently to form a new substance of matter – water. Yet, the total mass remains the same, once again proving the law of conservation of matter.
Schedule a Free session to clear worksheet doubts
No credit card required, no obligation to purchase.
Just schedule a FREE Sessions to meet a tutor and get help on any topic you want!
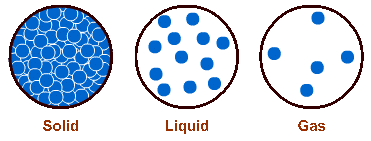
Solids, for example, are packed densely with molecules – these molecules do not move from one part of the matter to another. They have a definite shape and they occupy a definite space.
In liquids, molecules move about a bit more easily, but yet not far apart from one another. Liquids, therefore, have an indefinite shape but they still occupy a definite space.
Gas molecules move far apart from one another, they collide and repel one another and fill up all the space available to them, such as a jar containing ammonia gas. They, therefore, have no shape at all, and consequently they occupy indefinite space.
Volume and Density
The amount of space occupied by an object is called its Volume. The amount of mass fitted into a particular volume determines the Density of that matter.
In other words, Density = Mass/Volume.
Solids have higher density than liquids – their molecules are closer together – and liquids have higher density than gases. Therefore, for the same amount of mass, solids tend to occupy lesser space than liquids and liquids occupy lesser space than gases.
Learn more about Conservation of Matter and other important topics with 8th Grade Science Tutoring at eTutorWorld. Our expert science tutors break down the topics through interactive one-to-one sessions. We also offer the advantage of customized lesson plans, flexible schedules and convenience of learning from home.
eTutorWorld Understands Math Tutoring | Online Math Worksheets are Important Tools
Understanding graphs, charts, and opinion polls in a newspaper, for calculating house and car payments, and for choosing a long-distance telephone service are impossible without strong math skills …and the only way to develop strong math skills is by constant practice.
‘Practice makes a man perfect’ holds true for no other field better than for math. A middle or high school student must set aside a minimum of an hour for math every day. Other than textbooks, worksheets help you revise and understand concepts better.
Our expert tutors prepare online maths worksheets that are age and grade-appropriate. Grade-wise math worksheets for Elementary Math, Arithmetic, Pre-Algebra, Algebra, Geometry, Trigonometry, Statistics, Pre-Calculus and Calculus can be solved to improve math skills, to get ahead or to even catch up.
You may download these FREE online math worksheets in the PDF format, and then print and email us their solutions for a free evaluation and analysis by eTutorworld’smath expert tutors.
You may solve these worksheets by yourself or with your peers while studying together.
The Answer Key at the end of each worksheet allows for a self-evaluation.
Personalized Online Tutoring
eTutorWorld offers affordable one-on-one live tutoring over the web for Grades K-12, Test Prep help for Standardized tests like SCAT, CogAT, MAP, SSAT, SAT, ACT, ISEE and AP. You may schedule online tutoring lessons at your personal scheduled times, all with a Money-Back Guarantee. The first one-on-one online tutoring lesson is always FREE, no purchase obligation, no credit card required.
For answers/solutions to any question or to learn concepts, take a FREE CLASS.
No credit card required, no obligation to purchase.
Just book a free class to meet a tutor and get help on any topic you want!
A balanced chemical equation for the combustion of methane with the same number of atoms of elements before and after the reaction, demonstrating the law of conservation of matter.
Check Point
- The amount of matter in an object determines its _____.
- The amount of space occupied by an object is known as its ______.
- The amount of mass fitted into a particular volume is called its ______.
- For the same amount of mass, the matter having the least density would be –
- Liquid
- Gas
- Solid
- ______ can neither be created nor destroyed as a result of a physical or chemical change.
Answer Key
- Mass
- Volume
- Density
- b) Gas
- Matter
What is the law of conservation of matter?
The law of conservation of matter states that in a closed system, the total mass of the substances involved in a chemical reaction remains constant. Matter cannot be created or destroyed; it can only change forms or be rearranged.
Does the law of conservation of matter apply to all chemical reactions?
Yes, the law of conservation of matter applies to all chemical reactions that occur in a closed system. Whether it is a simple reaction or a complex process, the total mass of the substances involved remains constant.
What happens if the law of conservation of matter is violated in a reaction?
If the law of conservation of matter is violated in a reaction, it means that mass is either being created or destroyed, which contradicts our current understanding of chemistry. Such violations are not observed in nature or laboratory experiments and would require a modification of the existing understanding of matter and its behavior.
How is the law of conservation of matter related to balancing chemical equations?
Balancing chemical equations is the process of ensuring that the number of atoms of each element is the same on both sides of the equation. By balancing equations, we maintain the total mass of the reactants equal to the total mass of the products, adhering to the law of conservation of matter.
Schedule a Free session to clear worksheet doubts
No credit card required, no obligation to purchase.
Just schedule a FREE Sessions to meet a tutor and get help on any topic you want!
Pricing for Online Tutoring
| Tutoring Package | Validity | Grade (1-12), College |
|---|---|---|
| 5 sessions | 1 Month | $139 |
| 1 session | 1 Month | $28 |
| 10 sessions | 3 months | $269 |
| 15 sessions | 3 months | $399 |
| 20 sessions | 4 months | $499 |
| 50 sessions | 6 months | $1189 |
| 100 sessions | 12 months | $2249 |
8th Grade Free Worksheets
- The Universe
- Heredity
- Evolutionary Theory
- Structure of the atom
- Ethical Practices
- Unveiling the mystery behind the physical universe
- Components of the universe
- Celestial phenomena
- The tilt of Earth’s axis
- The causes of high and low tides
- Earth Systems
- Rocks and Fossils
- Weather and Climate
- Basics of chemical reactions
- Types of Chemical reactions – Endothermic, exothermic, oxidation, reduction reactions
- Catalysts and enzymes
- Compounds and mixtures
- Acids, Bases and pH Indicators
Images Credit:
https://pngimage.net/wp-content/uploads/2018/06/matter-png-5.png
https://upload.wikimedia.org/wikipedia/commons/7/7c/Combustion_reaction_of_methane.jpg
IN THE NEWS

Our mission is to provide high quality online tutoring services, using state of the art Internet technology, to school students worldwide.
Online test prep and practice
SCAT
SSAT
ISEE
PSAT
SAT
ACT
AP Exam
Science Tutoring
Physics Tutoring
Chemistry Tutoring
Biology Tutoring
Math Tutoring
Pre-Algebra Tutoring
Algebra Tutoring
Pre Calculus Tutoring
Calculus Tutoring
Geometry Tutoring
Trigonometry Tutoring
Statistics Tutoring
Quick links
Free Worksheets
Fact sheet
Sales Partner Opportunities
Parents
Passive Fundraising
Virtual Fundraising
Our Expert Tutors
Safe and Secure Tutoring
Interactive Online Tutoring
After School Tutoring
Elementary School Tutoring
Middle School Tutoring
High School Tutoring
Home Work Help
Math Tutors New York City
Press
©2022 eTutorWorld Terms of use Privacy Policy Site by Little Red Bird
©2022 eTutorWorld
Terms of use
Privacy Policy
Site by Little Red Bird






