Elements and Compounds Worksheets (Featuring Definitions, Difference, and Examples)
Science Worksheet for 7th Graders
An element is a pure substance made up of only one type of atom. Each element has its unique set of properties and is represented by a specific symbol. On the other hand, a compound is a substance composed of two or more elements chemically combined in fixed proportions.
Compounds have distinct properties that are different from those of their constituent elements. Understanding the characteristics and differences between elements and compounds is crucial in unraveling the complexities of the world around us.
Jump straight to Elements and Compounds Science Worksheets for 7th Graders. Click here.
The worksheets consists of multiple choice questions, give-one-word-answer and match-the-following which makes it more interesting to solve. These worksheets, and many more have been created by expert grade 7 science tutors who understand the importance of practice and questioning in the education of STEM subjects.
Learn more about Elements and Compounds and other important topics with 7th Grade Science Tutoring at eTutorWorld. Our expert science tutors break down the topics through interactive one-to-one sessions. We also offer the advantage of customized lesson plans, flexible schedules and convenience of learning from home.
Schedule a Free session to clear worksheet doubts
No credit card required, no obligation to purchase.
Just schedule a FREE Sessions to meet a tutor and get help on any topic you want!
Elements
Everything around us is made up of elements. Let’s take the air we breathe is mixture so many elements like oxygen, nitrogen, and argon. They are the constituting factors for everything on this planet Earth: the tree, your book, your pencil, paper, and furniture, so on.
An element is a pure substance that cannot be broken down by chemical methods into simpler parts.
For example
- A piece of iron cannot be broken down into anything other than iron. If you kept breaking that piece, the pieces would get smaller, but each piece will always be iron.

- A copper wire is an example of an element/ pure substance. The more we break it down, more pieces of copper we get.
Elements consist of only one type of atom. An atom is the smallest particle of an element that still has the same properties of that element. All atoms of a specific element have exactly the same chemical makeup, size, and mass.
There are a total of 118 elements.
Many elements occur naturally on Earth; however, some are created in a laboratory by scientists by nuclear processes.
eTutorWorld Understands Math Tutoring | Online Math Worksheets are Important Tools
Understanding graphs, charts, and opinion polls in a newspaper, for calculating house and car payments, and for choosing a long-distance telephone service are impossible without strong math skills …and the only way to develop strong math skills is by constant practice.
‘Practice makes a man perfect’ holds true for no other field better than for math. A middle or high school student must set aside a minimum of an hour for math every day. Other than textbooks, worksheets help you revise and understand concepts better.
Our expert tutors prepare online maths worksheets that are age and grade-appropriate. Grade-wise math worksheets for Elementary Math, Arithmetic, Pre-Algebra, Algebra, Geometry, Trigonometry, Statistics, Pre-Calculus and Calculus can be solved to improve math skills, to get ahead or to even catch up.
You may download these FREE online math worksheets in the PDF format, and then print and email us their solutions for a free evaluation and analysis by eTutorworld’smath expert tutors.
You may solve these worksheets by yourself or with your peers while studying together.
The Answer Key at the end of each worksheet allows for a self-evaluation.
Personalized Online Tutoring
eTutorWorld offers affordable one-on-one live tutoring over the web for Grades K-12, Test Prep help for Standardized tests like SCAT, CogAT, MAP, SSAT, SAT, ACT, ISEE and AP. You may schedule online tutoring lessons at your personal scheduled times, all with a Money-Back Guarantee. The first one-on-one online tutoring lesson is always FREE, no purchase obligation, no credit card required.
For answers/solutions to any question or to learn concepts, take a FREE CLASS.
No credit card required, no obligation to purchase.
Just book a free class to meet a tutor and get help on any topic you want!
Compounds
Every combination of atoms is a molecule. Numerous atoms of different elements combine to form a compound molecule. All compounds are molecules but all molecules are not compounds. Molecule is one that is formed together out of a chemical bond.
- Sodium (Na) is a molecule, but not a compound because it is made of only one element.
- Common Salt (NaCl) can be called a molecule or a compound because it is made of Sodium Na, and Chlorine Cl.
- One atom of carbon + one molecule of oxygen = one molecule of carbon dioxide.
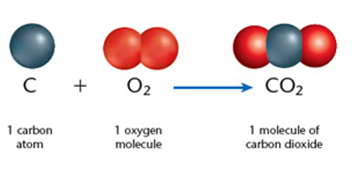
An illustration of how element compound and mixture looks like:
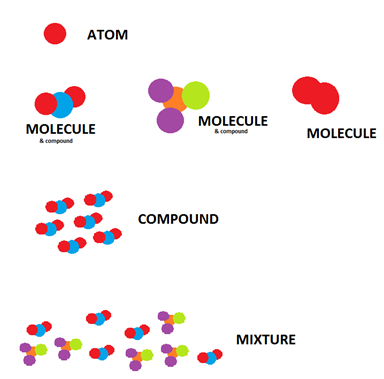
Credit: https://chemistry.stackexchange.com/questions/2879/what-is-the-definition-of-of-compound-mixture-element-and-molecule
Schedule a Free session to clear worksheet doubts
No credit card required, no obligation to purchase.
Just schedule a FREE Sessions to meet a tutor and get help on any topic you want!
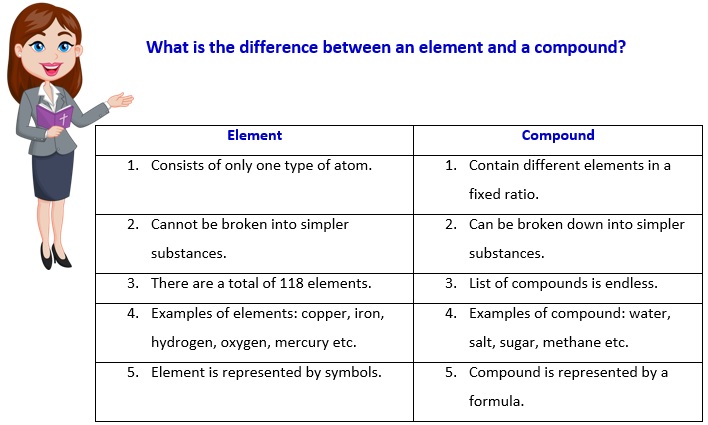
What elements and compounds have in common?
Both, elements and compound are pure chemical substances.
Physical changes: A change which does not affect the substance. Physical forces cannot always break down compounds totally. Heat melts ice to form water. But no change happens to the molecule of water. Like -wise decreasing the pressure boils water leaving the molecules unchanged.
Chemical changes in compounds happen when chemical bonds are created or destroyed.
In a chemical change the forces between atoms, changes the molecular pattern of a substance. Energy is either released or absorbed in the process.
The process of curdling of milk is also an example of chemical change.
- A new substance is formed
- The process is irreversible
There are millions of different compounds around us. When elements join and become compounds, they lose many of their individual behavior. Sodium (Na) is very reactive element individually but when sodium and chlorine (Cl) combine, table salt forms which is a non-reactive and obviously very edible substance. New compounds formed rarely posses any of their previous physical or chemical traits when they were elements. They have a new life of their own.
Check Point
A. Fill in the blanks:
- An …………… Is a pure substance that cannot be broken down by chemical methods into simpler components.
- An …………… is the smallest particle of an element that still has the same properties of that element.
- There are a total of …………… elements.
- A …………… is a molecule made of atoms from different elements.
- …………… …………… in compounds happen when chemical bonds are created or destroyed.
B. State True or False:
- All atoms of a specific element have the different chemical makeup, size, and mass.
- Molecule is used to describe any atoms that are together by a chemical bond.
- Every compound is a molecule, and every molecule is a compound.
- When elements join and become compounds, they do not lose many of their individual behaviors.
- Physical forces alone rarely break down compounds completely.
Answer Key
A. Fill in the blanks:
- Element
- Atom
- 118
- Compound
- Chemical Changes
B. State True or False
- False
- True
- False
- False
- True
Sample Questions
Q1. One Word Answer:
- It takes the shape of the container._____________
- It is made up of particles/atoms of only one kind._____________
- A Compound is same throughout in properties and composition. We call it _________________
- A Smallest unit of an element that has all the basic properties of the element._________________
- It is made up of two or more kinds of atoms or compounds mixed in any proportion.________________
- This molecule is made of two atoms of hydrogen and one atom of oxygen._______________
- One example of an element. ________________
- The elements are placed in specific locations because of the way they look and act. _________________
- These substances will have a constant appearance, color and density throughout the sample. ________________
- Objects that take up space and have mass._________________
Learn more about Elements and Compounds and other important topics with 7th Grade Science Tutoring at eTutorWorld. Our expert science tutors break down the topics through interactive one-to-one sessions. We also offer the advantage of customized lesson plans, flexible schedules and convenience of learning from home.
What are elements?
Elements are pure substances made up of only one type of atom. They cannot be broken down into simpler substances by chemical means.
What are compounds?
Compounds are substances formed by chemically combining two or more elements in fixed proportions. They have unique properties different from their constituent elements.
How are elements and compounds different?
The main difference between elements and compounds is their composition. Elements consist of only one type of atom, while compounds are composed of different types of atoms chemically bonded together.
How are elements and compounds represented?
Elements are represented by symbols, usually derived from their names. For example, oxygen is represented by the symbol O. Compounds are represented using chemical formulas that indicate the types and numbers of atoms present in the compound.
Are there any free worksheets available for practicing elements and compounds?
Yes, our website provides free worksheets for grade 7 students to practice and reinforce their understanding of elements and compounds. These worksheets offer a variety of exercises to enhance their knowledge and skills in this area of chemistry.
Pricing for Online Tutoring
| Tutoring Package | Validity | Grade (1-12), College |
|---|---|---|
| 5 sessions | 1 Month | $139 |
| 1 session | 1 Month | $28 |
| 10 sessions | 3 months | $269 |
| 15 sessions | 3 months | $399 |
| 20 sessions | 4 months | $499 |
| 50 sessions | 6 months | $1189 |
| 100 sessions | 12 months | $2249 |
IN THE NEWS

Our mission is to provide high quality online tutoring services, using state of the art Internet technology, to school students worldwide.
Online test prep and practice
SCAT
CogAT
SSAT
ISEE
PSAT
SAT
ACT
AP Exam
Science Tutoring
Physics Tutoring
Chemistry Tutoring
Biology Tutoring
Math Tutoring
Pre-Algebra Tutoring
Algebra Tutoring
Pre Calculus Tutoring
Calculus Tutoring
Geometry Tutoring
Trigonometry Tutoring
Statistics Tutoring
Quick links
Free Worksheets
Fact sheet
Sales Partner Opportunities
Parents
Passive Fundraising
Virtual Fundraising
Our Expert Tutors
Safe and Secure Tutoring
Interactive Online Tutoring
After School Tutoring
Elementary School Tutoring
Middle School Tutoring
High School Tutoring
Home Work Help
Math Tutors New York City
Press
©2022 eTutorWorld Terms of use Privacy Policy Site by Little Red Bird
©2022 eTutorWorld
Terms of use
Privacy Policy
Site by Little Red Bird






