What is Thermal Energy?
Grade 6 Science Worksheets
Thermal energy is a type of energy that results from the movement of particles within a substance. It is the sum of the kinetic energy of all the particles in a substance, including atoms, molecules, and ions.
Table of Contents:
- Thermal Energy
- Thermal Energy Production
- Thermal Energy Storage
- Thermal Energy and Molecules
- FAQs
What is Thermal Energy? - Grade 6 Science Worksheet PDF
This is a free printable / downloadable PDF worksheet with practice problems and answers. You can also work on it online.
|
Untimed | |
Sign up with your email ID to access this free worksheet.
"We really love eTutorWorld!"
"We really love etutorworld!. Anand S and Pooja are excellent math teachers and are quick to respond with requests to tutor on any math topic!" - Kieran Y (via TrustSpot.io)
"My daughter gets distracted easily"
"My daughter gets distracted very easily and Ms. Medini and other teachers were patient with her and redirected her back to the courses.
With the help of Etutorworld, my daughter has been now selected in the Gifted and Talented Program for the school district"
- Nivea Sharma (via TrustSpot.io)
THERMAL ENERGY
In other words, thermal energy is the energy that a substance possesses due to its temperature.
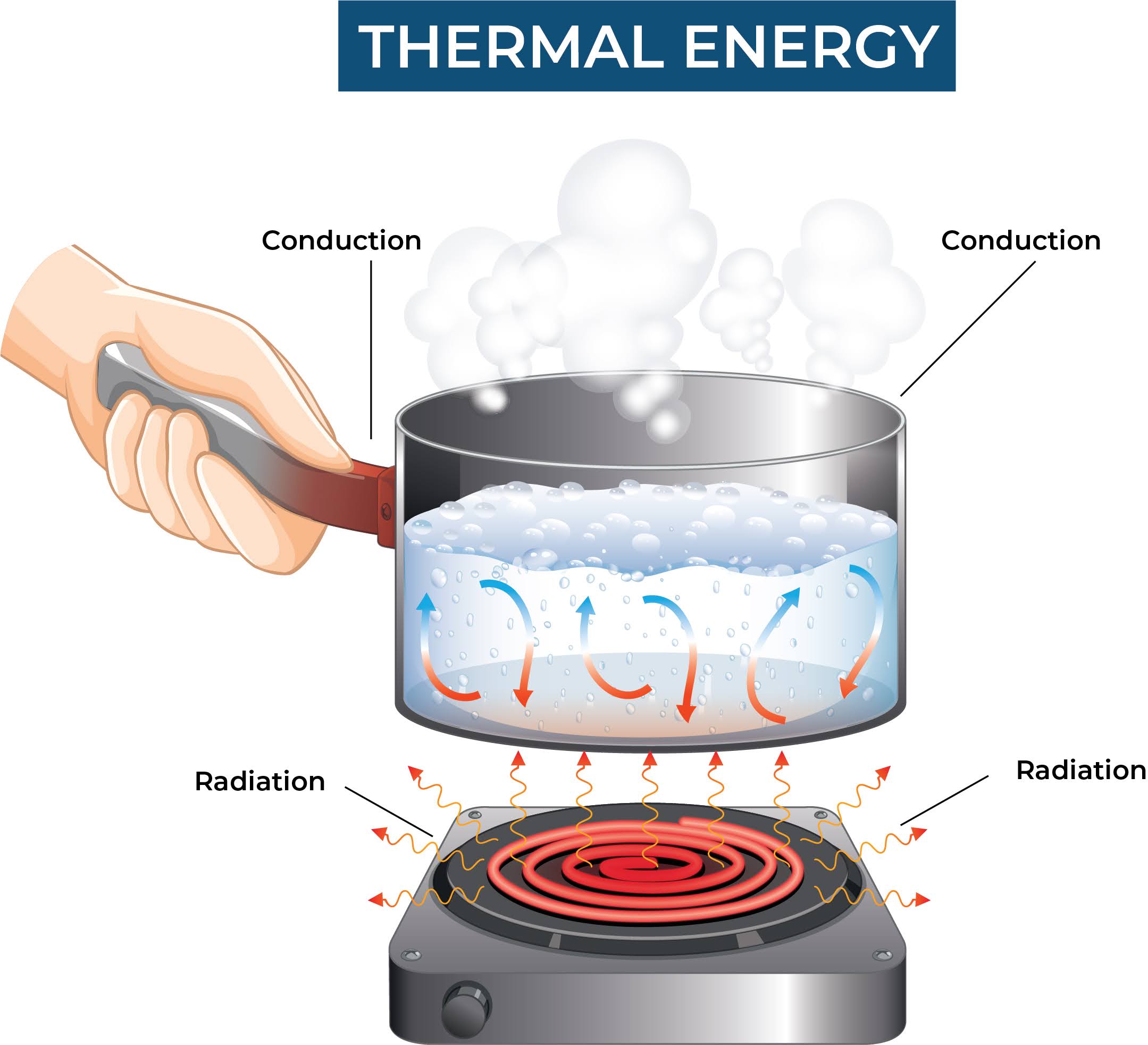
The faster the particles move within a substance, the more thermal energy it has. When two substances are in contact with each other, heat can be transferred from the substance with higher thermal energy to the substance with lower thermal energy, until both substances reach the same temperature.
Thermal energy is a fundamental concept in thermodynamics, which is the study of the relationship between heat, work, and energy. It has many practical applications, such as in the design and operation of engines, refrigeration systems, and heating systems.
THERMAL ENERGY PRODUCTION
Thermal energy can be produced in a variety of ways, but generally it is produced through the conversion of other forms of energy, such as chemical, nuclear, or mechanical energy, into heat. Here are some examples:
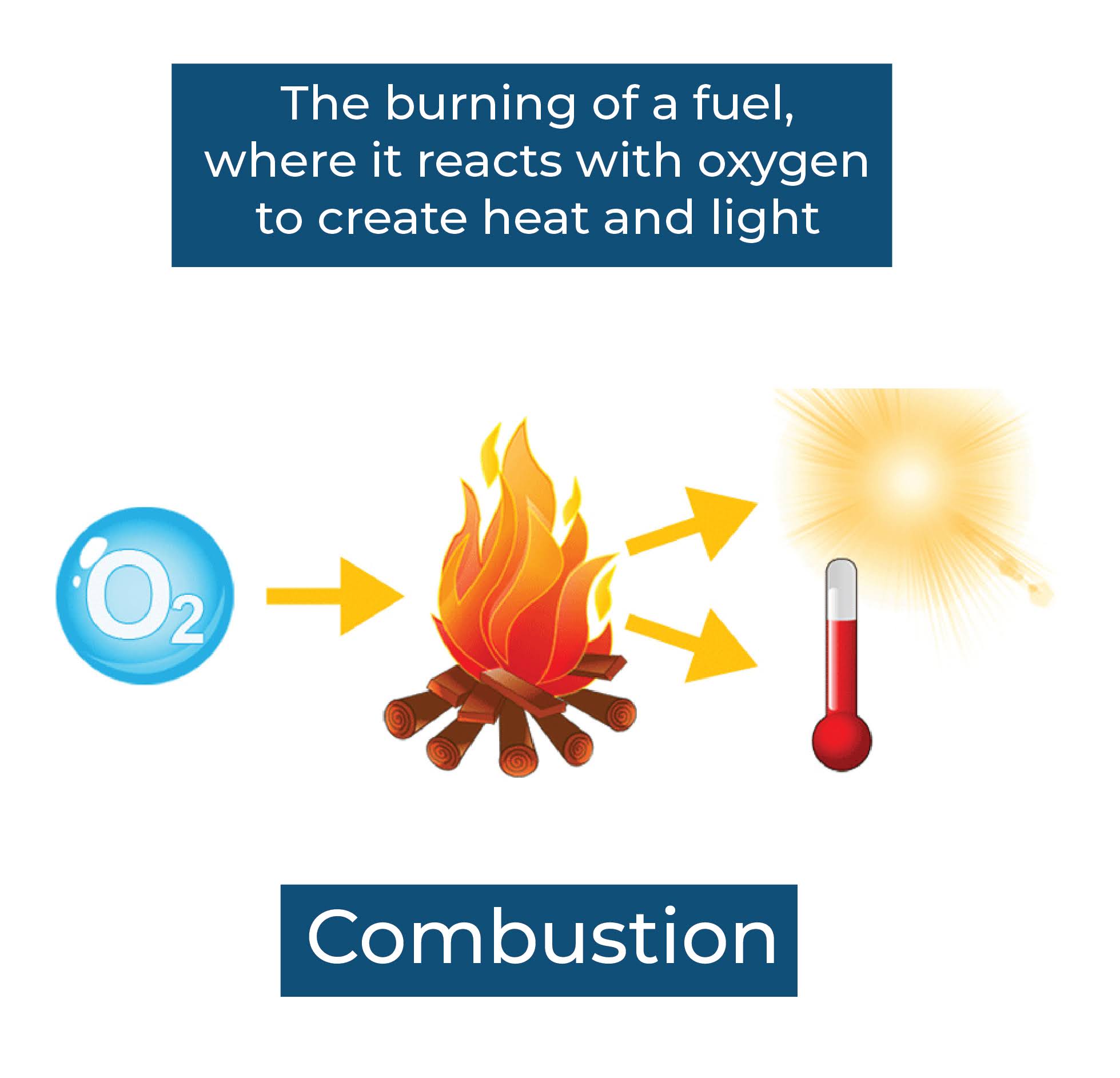
Combustion: Thermal energy can be produced through the combustion of fossil fuels, such as coal, oil, or natural gas. When these fuels are burned, the chemical energy in the fuel is converted into heat.
Nuclear fission: Thermal energy can be produced through nuclear fission, which is the process of splitting atoms in a nuclear reactor. This releases a large amount of energy in the form of heat.
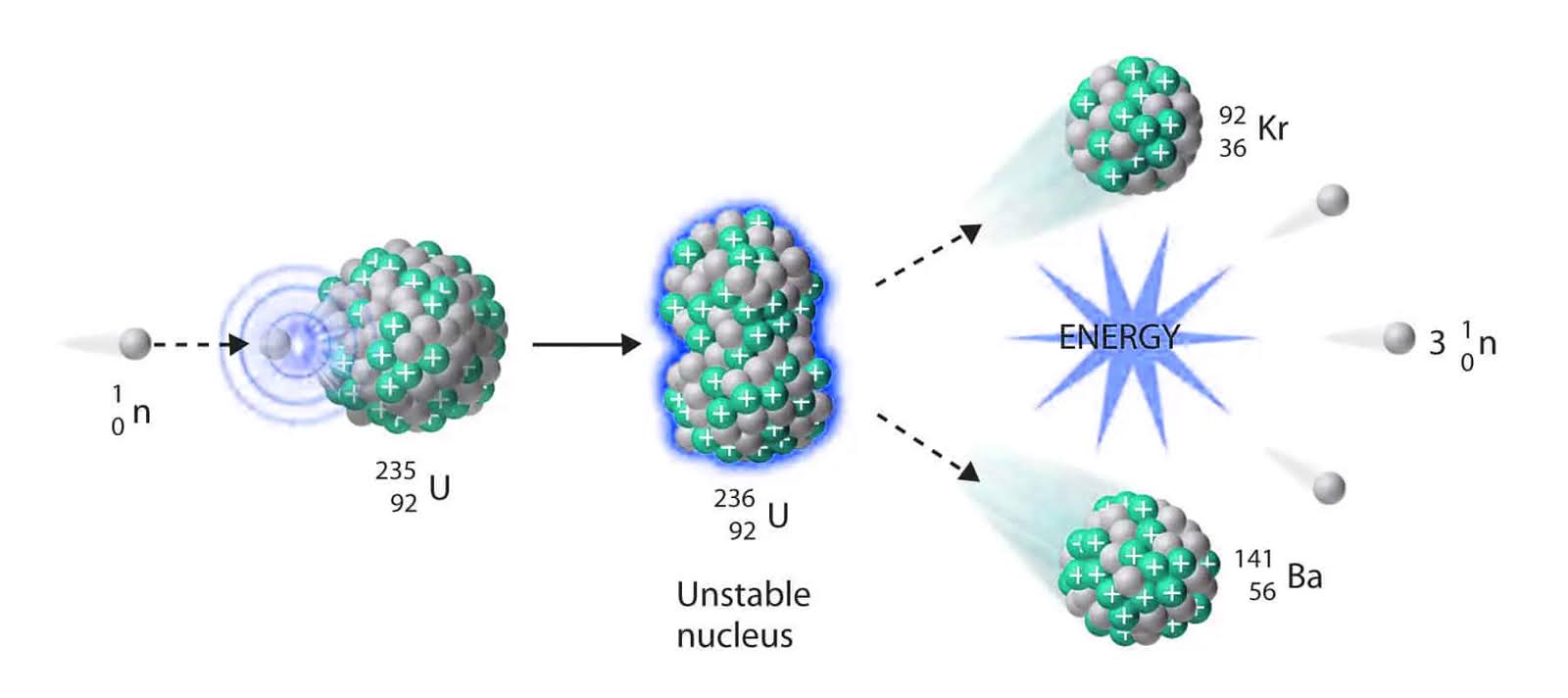
Solar energy: Thermal energy can be produced through the absorption of sunlight. When sunlight is absorbed by a surface, such as a solar panel or a building, the energy is converted into heat.

Friction: Thermal energy can be produced through friction, which is the force that opposes motion between two surfaces. When two surfaces rub against each other, the friction generates heat.
Electrical resistance: Thermal energy can be produced through electrical resistance, which is the property of a material that resists the flow of electrical current. When current flows through a material with high resistance, such as a resistor, the electrical energy is converted into heat.
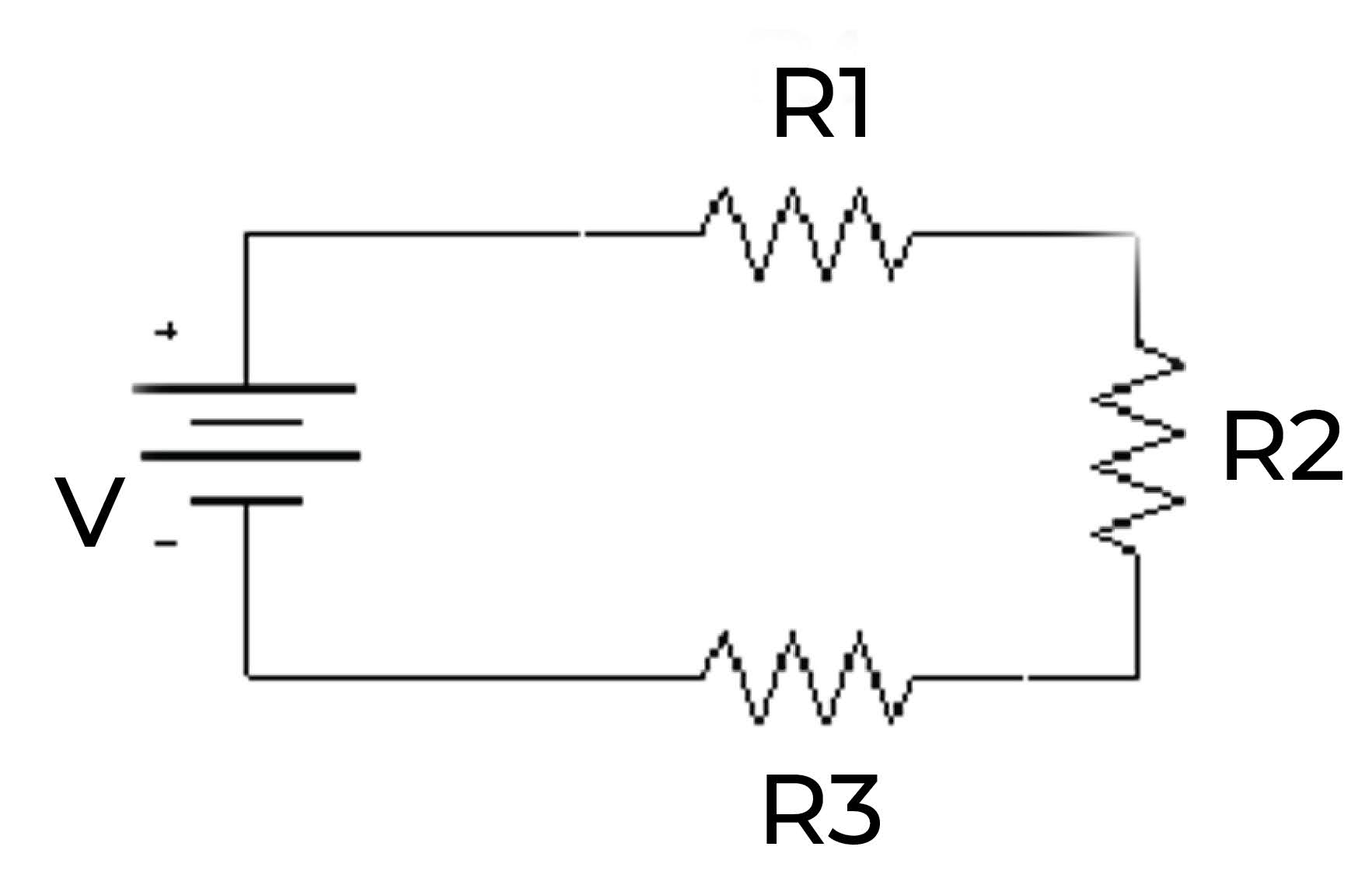
In all of these cases, the energy is ultimately converted into heat, which is the form of energy that is associated with thermal energy.
THERMAL ENERGY STORAGE
Thermal energy storage (TES) is a process by which thermal energy is stored for later use, typically during times when energy demand is high or when the source of energy is intermittent. TES allows energy to be stored when it is available and then used when it is needed, which can help to improve the efficiency and reliability of energy systems.
There are several types of thermal energy storage systems, including:
Sensible heat storage: This involves storing thermal energy in the temperature of a substance, such as water or rocks.
Latent heat storage: This involves storing thermal energy in the phase change of a substance, such as melting or freezing.
Thermochemical storage: This involves storing thermal energy in the chemical bonds of a substance, which can be released through a chemical reaction.
TES is used in a variety of applications, including heating and cooling buildings, generating electricity, and industrial processes. For example, a building might use TES to store thermal energy during off-peak hours, when energy is less expensive, and then use that stored energy to provide heating or cooling during peak demand periods. TES can also be used to store thermal energy from renewable sources, such as solar or wind power, which can be intermittent.
TES systems can help to improve the efficiency of energy systems and reduce costs, while also helping to reduce greenhouse gas emissions by increasing the use of renewable energy sources.
One additional concept related to thermal energy is heat transfer, which is the movement of thermal energy from one object or substance to another due to a difference in temperature. There are three main mechanisms of heat transfer:
Conduction: Heat transfer by direct contact between two objects or substances. In this process, heat is transferred from the hotter object to the cooler object.
Convection: Heat transfer through the movement of fluids, such as liquids or gases. In this process, heat is transferred from one region of the fluid to another by the movement of the fluid itself.
Radiation: Heat transfer through electromagnetic waves, such as infrared radiation. In this process, heat is transferred from one object to another without the need for direct contact or a medium to carry the heat.
Heat transfer is an important concept in many fields, including thermodynamics, engineering, and environmental science. Understanding how heat is transferred is crucial for designing efficient energy systems, developing effective insulation materials, and studying climate change, among other applications.
“There have been times when we booked them last minute, but the teachers have been extremely well-prepared and the help desk at etutorworld is very prompt.
Our kid is doing much better with a higher score.”
6th Grade Tutoring
eTutorWorld offers Personalized Online Tutoring for Math, Science, English, and Standardised Tests.
Our Tutoring Packs start at just under $22.49 per hour, and come with a moneyback guarantee.
Schedule a FREE Trial Session, and experience quality tutoring for yourself. (No credit card required.)
THERMAL ENERGY & MOLECULES
In a substance, such as a gas or a liquid, the individual molecules are in constant motion, with each molecule moving at a particular speed and in a particular direction. This motion is known as molecular motion, and it is the result of the thermal energy in the substance.
The temperature of a substance is a measure of the average kinetic energy of the molecules in the substance. At higher temperatures, the molecules have a higher average kinetic energy and move faster, while at lower temperatures, the molecules have a lower average kinetic energy and move slower.
When a substance is heated, the molecules in the substance absorb thermal energy and their average kinetic energy increases. This causes the molecules to move faster and collide more frequently with other molecules. As a result, the substance becomes hotter.
Conversely, when a substance is cooled, the molecules in the substance lose thermal energy and their average kinetic energy decreases. This causes the molecules to move more slowly and collide less frequently with other molecules. As a result, the substance becomes colder.
It’s worth noting that in a substance, there are always molecules with different speeds and kinetic energies. Some molecules will be moving faster and have more kinetic energy, while others will be moving slower and have less kinetic energy. When we say that a substance is “hot” or “cold,” we are referring to the average kinetic energy of the molecules in the substance.
The specific heat capacity of a substance also plays a role in how much thermal energy is required to change the temperature of the substance.
The specific heat capacity is the amount of thermal energy required to raise the temperature of one unit of mass of a substance by one degree Celsius.
Substances with a higher specific heat capacity require more thermal energy to increase their temperature compared to substances with a lower specific heat capacity.
Overall, the movement of molecules and their kinetic energy are closely linked to thermal energy and temperature, and understanding these concepts is crucial in many areas of science and engineering, including thermodynamics, heat transfer, and materials science.
Do You Stack Up Against the Best?
If you have 30 minutes, try our free diagnostics test and assess your skills.
FAQS
What is thermal energy?
Thermal energy is the energy that is generated by the movement of particles within a substance. It is a form of kinetic energy that is associated with the random motion of molecules and atoms.
How is thermal energy measured?
Thermal energy is typically measured in joules (J) or calories (cal), and is usually determined using a thermometer or other temperature measuring device.
What are some common sources of thermal energy?
Some common sources of thermal energy include the sun, geothermal heat from the earth, and combustion of fossil fuels.
How is thermal energy transferred?
Thermal energy can be transferred from one substance to another through conduction, convection, or radiation. In conduction, heat is transferred through direct contact between two substances. In convection, heat is transferred through the movement of a fluid or gas. In radiation, heat is transferred through electromagnetic waves.
What are some applications of thermal energy?
Thermal energy is used in a wide variety of applications, including heating homes and buildings, generating electricity, cooking food, and powering engines.
What is the difference between thermal energy and temperature?
Temperature is a measure of the average kinetic energy of the molecules in a substance, while thermal energy is the total kinetic energy of all the molecules in a substance. The higher the temperature, the greater the thermal energy.
Can thermal energy be converted into other forms of energy?
Yes, thermal energy can be converted into other forms of energy, such as mechanical energy, electrical energy, or light energy. For example, thermal energy can be used to power a steam turbine, which in turn generates electricity.

Kathleen Currence is one of the founders of eTutorWorld. Previously a middle school principal in Kansas City School District, she has an MA in Education from the University of Dayton, Ohio. She is a prolific writer, and likes to explain Science topics in student-friendly language. LinkedIn Profile
Affordable Tutoring Now Starts at Just $22.49
eTutorWorld offers affordable one-on-one live tutoring over the web for Grades K-12. We are also a leading provider of Test Prep help for Standardized Tests (SCAT, CogAT, MAP, SSAT, SAT, ACT, ISEE, and AP).
What makes eTutorWorld stand apart are: flexibility in lesson scheduling, quality of hand-picked tutors, assignment of tutors based on academic counseling and diagnostic tests of each student, and our 100% money-back guarantee.
Whether you have never tried personalized online tutoring before or are looking for better tutors and flexibility at an affordable price point, schedule a FREE TRIAL Session with us today.
*There is no purchase obligation or credit card requirement
Grade 6 Science Worksheets
- Inquiry process
- Nature of Science
- Scientific Inquiry
- Inquiry, Analysis and Problem Solving
- Ethical Practices
- Science and Society
- Biotic and Abiotic Factors
- Impact of Organisms
- Adaptation
- Spheres of Earth
- Natural Resources
- Environmental Issues
- Conservation of Earth
- Understanding Technology
- Abilities To Do Technological Design
- Structure of Earth
- Solar System
- Rocks and Fossils
- Earth Systems
- Plate Tectonics
- Evolution
- Magnetic Field of Earth
- Geologic Time
- Materials and Processes That Shape a Planet
- Astronomy
- Ecology
- Energy
- Kinetic and Potential Energy
- Energy Transfer
- Matter and its Structure
- States of Matter
- Physical and Chemical Changes
- Force and Motion
- Electricity and Magnetism
- Wave Interactions
- Sound
- Light
- Introduction to Life Science
- The Origin & History of Life On Earth
- Plant and Animal Cells
- Parts of a Cell
- The Cell Cycle
- How Living Organisms Get Energy
- Classification of Organisms
- How Plants Grow & Reproduce
- The Human Respiratory System
- The Human Cardiovascular System
- The Human Digestive System
- The Human Endocrine Systems
- The Human Nervous System
- The Human Muscular System
- The Human Skeletal System
IN THE NEWS

Our mission is to provide high quality online tutoring services, using state of the art Internet technology, to school students worldwide.
Online test prep and practice
SCAT
CogAT
SSAT
ISEE
PSAT
SAT
ACT
AP Exam
Science Tutoring
Physics Tutoring
Chemistry Tutoring
Biology Tutoring
Math Tutoring
Pre-Algebra Tutoring
Algebra Tutoring
Pre Calculus Tutoring
Calculus Tutoring
Geometry Tutoring
Trigonometry Tutoring
Statistics Tutoring
Quick links
Free Worksheets
Fact sheet
Sales Partner Opportunities
Parents
Passive Fundraising
Virtual Fundraising
Our Expert Tutors
Safe and Secure Tutoring
Interactive Online Tutoring
After School Tutoring
Elementary School Tutoring
Middle School Tutoring
High School Tutoring
Home Work Help
Math Tutors New York City
Press
©2022 eTutorWorld Terms of use Privacy Policy Site by Little Red Bird
©2022 eTutorWorld
Terms of use
Privacy Policy
Site by Little Red Bird