What is Evaporation?
Grade 6 Science Worksheets
A liquid turns into a gas through the process of evaporation when the temperature rises or the pressure falls. The liquid particles gain kinetic energy and their intermolecular distances widen during evaporation, enabling them to elude detection and enter the gas phase.
Table of Contents:
- What is Evaporation?
- Is Evaporation a Chemical Process?
- Molecular Changes in Evaporation
- Can Solids be Evaporation?
- Can Evaporation be reversed?
- Uses of Evaporation
- Exceptions to Evaporation
- Experiments with Evaporation
- FAQs
What is Evaporation? - Grade 6 Science Worksheet PDF
This is a free printable / downloadable PDF worksheet with practice problems and answers. You can also work on it online.
|
Untimed | |
Sign up with your email ID to access this free worksheet.
"We really love eTutorWorld!"
"We really love etutorworld!. Anand S and Pooja are excellent math teachers and are quick to respond with requests to tutor on any math topic!" - Kieran Y (via TrustSpot.io)
"My daughter gets distracted easily"
"My daughter gets distracted very easily and Ms. Medini and other teachers were patient with her and redirected her back to the courses.
With the help of Etutorworld, my daughter has been now selected in the Gifted and Talented Program for the school district"
- Nivea Sharma (via TrustSpot.io)
The difference in vapour pressure between a liquid and its surroundings is what causes evaporation to happen at the liquid’s surface.
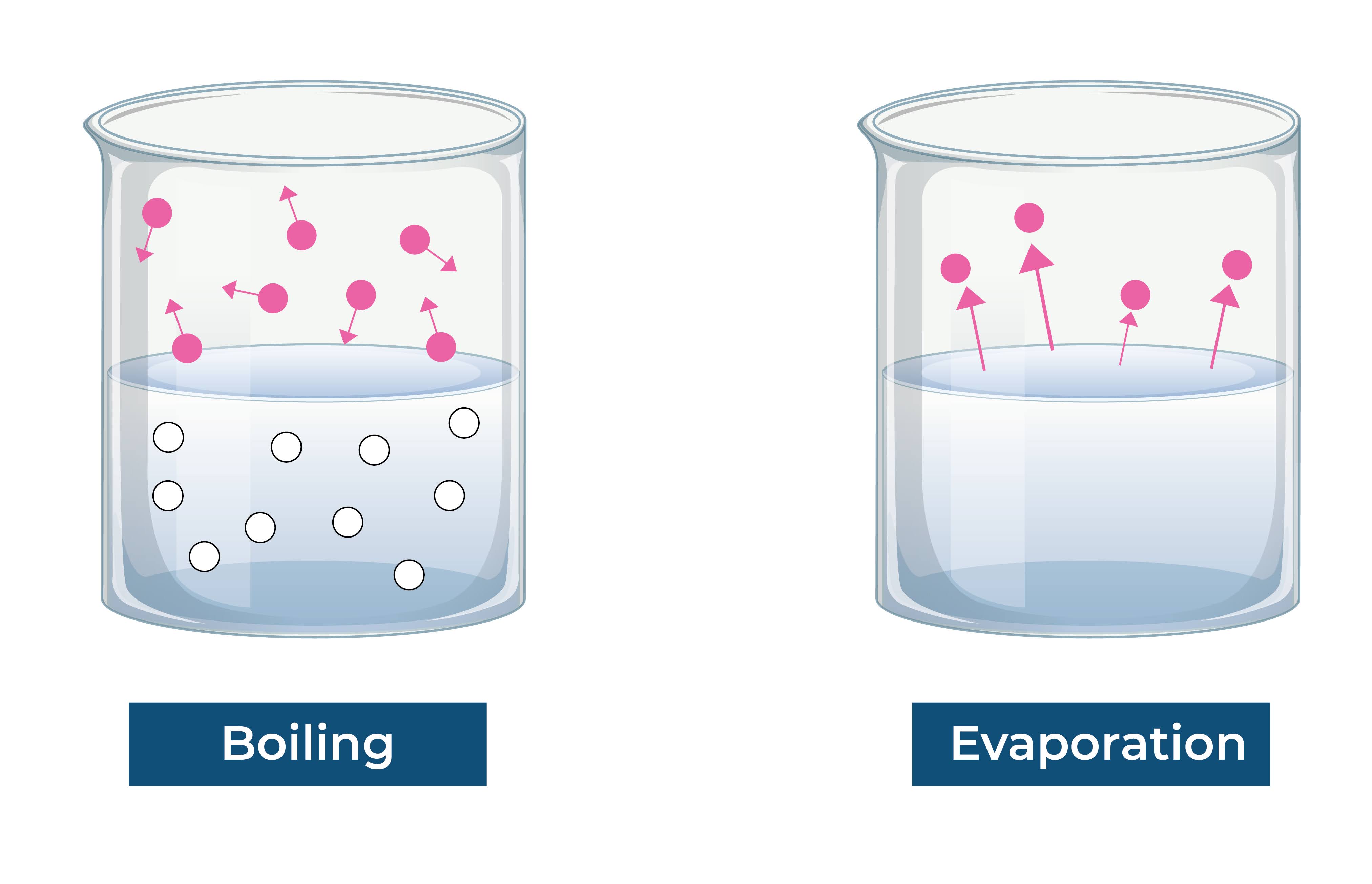
The temperature of the liquid, its surface area, the air movement over the liquid’s surface, and the relative humidity of the immediate environment all have an impact on the rate of evaporation.
The ocean, lakes, rivers, refrigeration systems, and other natural and industrial systems all experience evaporation on a regular basis. In many industrial processes, including distillation and drying, it is also used to separate liquids from solids or to dry out materials.
Is Evaporation a Chemical Process?
Evaporation does not result from a chemical reaction. When a liquid turns into a gas as a result of an increase in temperature or a decrease in pressure, it happens physically.
When water is heated on a stove and converted to steam, this is an instance of evaporation. The water molecules on the liquid’s surface gain kinetic energy as a result of the heat energy, erupt into the gas phase, and condense into steam.
The chemical bonds between the atoms or molecules in the water are unaffected by this process.
When water evaporates from a puddle on a sunny day, that is another instance of evaporation in action. The water molecules on the liquid’s surface gain kinetic energy and escape into the air as a result of the sun’s heat energy, which causes the puddle to shrink. The chemical makeup of the water is unaltered during this process.
Molecular changes in Evaporation
The liquid particles gain kinetic energy and their intermolecular distances widen during evaporation, enabling them to elude detection and enter the gas phase.
The difference in vapour pressure between a liquid and its surroundings is what causes evaporation to happen at the liquid’s surface.
Evaporation can be conceptualized at the molecular level as the transfer of energy from the environment to the liquid particles. The liquid particles gain kinetic energy and their intermolecular distances grow as the energy is absorbed by them. By doing so, they are able to defeat the forces of attraction between the particles and enter the gas phase.
Evaporation has no impact on the chemical bonds that connect the atoms or molecules in the molecules’ chemical structure. Evaporation doesn’t alter the chemical makeup of the substance; it only changes the physical state of the material from a liquid to a gas.
Can Solids be Evaporated?
The traditional definition of evaporation does not apply to solids. When a liquid turns into a gas as a result of an increase in temperature or a decrease in pressure, this process is known as evaporation.
Particles in solids are tightly packed together and lack enough kinetic energy to escape into the gas phase because they have a defined shape and volume.
Sublimation, on the other hand, is a process that can transform some solids into vapour. Bypassing the liquid phase, sublimation is the process by which a solid transforms instantly into a gas.
When a solid takes in enough energy to overcome the intermolecular forces holding its particles in place and allow them to escape into the gas phase, this process takes place.
Dry ice (solid carbon dioxide) and naphthalene are two examples of solids that can sublimate (mothballs). These solids won’t go through the liquid phase when they are exposed to air; instead, they will slowly transform into a gas.
In comparison to evaporation, this is a relatively slow process because the solid needs to absorb enough energy to dismantle the intermolecular forces holding its particles together.
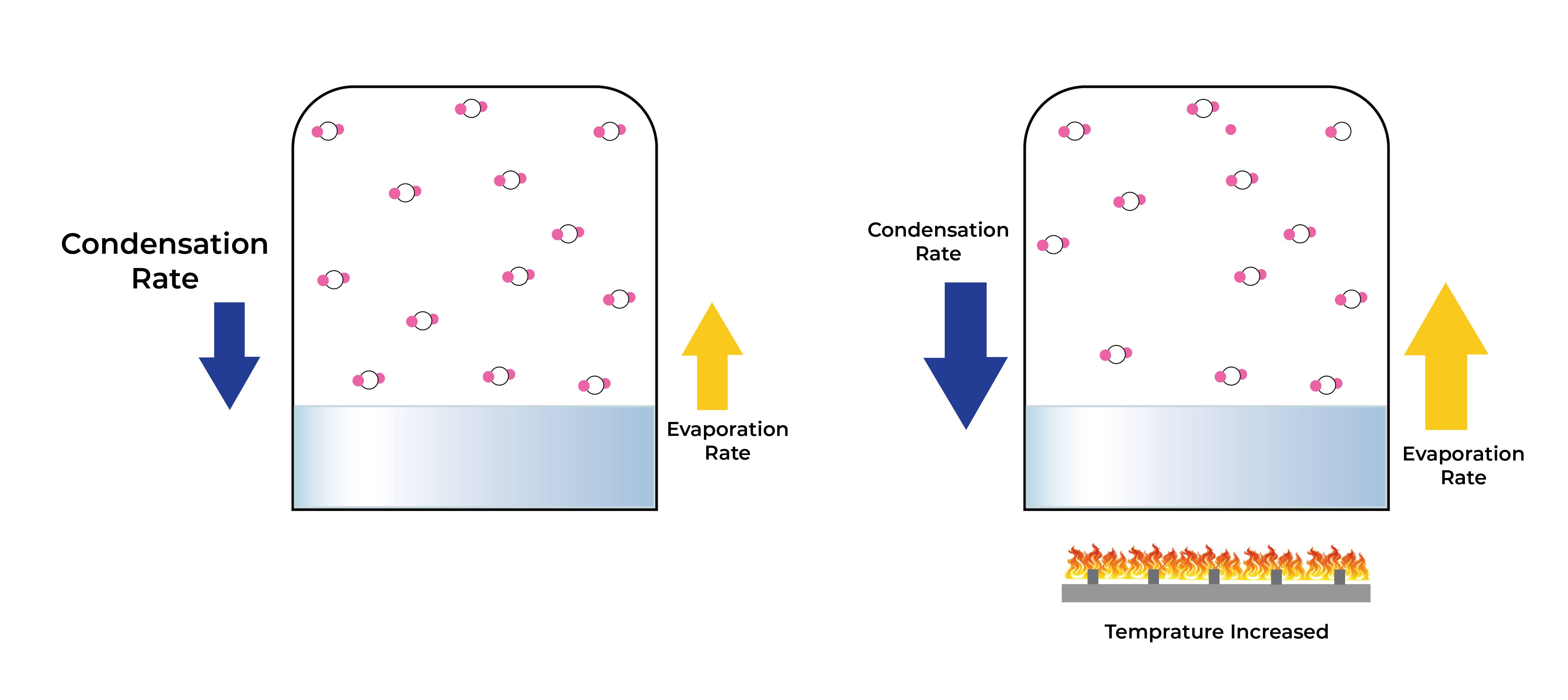
Can Evaporation be Reversed?
Yes, it is possible to stop evaporation by using the condensation process. Condensation is when a gas or vapour turns into a liquid, reversing the process of evaporation.
When the temperature of the gas or vapour is lowered to a level where the particles have sufficient energy to override their intermolecular forces and condense into a liquid, this process takes place.
When water evaporates, for instance, the water molecules change into water vapour and rise into the air. On a cold surface, like the inside of a glass on a hot day, water vapour can condense back into a liquid and form droplets if it cools down.
Condensation occurs frequently and is a significant process in many natural and man-made systems. It is crucial to the water cycle, cloud formation, and the production of dew and fog.
Condensation is a process that reverses the evaporation process, allowing liquids to be recovered and purified as well as gases to be condensed into liquids for storage or transportation.
“There have been times when we booked them last minute, but the teachers have been extremely well-prepared and the help desk at etutorworld is very prompt.
Our kid is doing much better with a higher score.”
6th Grade Tutoring
eTutorWorld offers Personalized Online Tutoring for Math, Science, English, and Standardised Tests.
Our Tutoring Packs start at just under $22.49 per hour, and come with a moneyback guarantee.
Schedule a FREE Trial Session, and experience quality tutoring for yourself. (No credit card required.)
Uses of Evaporation
Many different industries and applications use evaporation as a common process, including:
Evaporation is a technique used in water treatment facilities to get rid of dissolved solids from water. The solids are concentrated in the liquid phase as a result of heating the water, and the solids are then left behind as the water is evaporated.
1. Food and beverage production: Fruit juices, syrups, and concentrates are just a few of the products that are made using evaporation in the food and beverage industry. As the liquid is heated to remove extra water, the flavours and sugars are concentrated.
2. Pharmaceuticals: Evaporation is used to produce syrups, suspensions, and sprays, among other pharmaceutical products. In order to concentrate the active ingredients and get rid of any impurities, the liquid is evaporated.
3. Production of salt: The production of salt, including table salt and sea salt, uses evaporation. Seawater is pumped into evaporation ponds, where it is exposed to the sun and wind, where it evaporates, leaving behind the salt.
4. Evaporation: It is a key component in the desalination process, which removes salt from seawater to create fresh water. As a result of heating the seawater, the water evaporates, leaving the salt behind.
5. Waste treatment: In waste treatment facilities, evaporation is used to separate liquids from solid waste. The solid waste is left behind for disposal after the liquid has evaporated.
These are just a few instances of the many fields and applications where evaporation is used. Evaporation is a common, efficient, and easy way to get rid of liquids and concentrate liquids and solids.
EXCEPTIONS TO EVAPORATION
The evaporation process has a few exceptions, such as:
1. In some circumstances, a solute dissolved in a solvent can lower the solvent’s freezing point, resulting in a liquid that freezes before it can evaporate.
2. Similar to this, a solute dissolved in a solvent can raise the solvent’s boiling point, causing the liquid to boil at a higher temperature and delaying the evaporation process.
3. Supercooling is the process of cooling a liquid below its normal freezing point without it congealing. Supercooling is a technique that can stop a liquid from evaporating.
4. Glass transition temperature: In some substances, like amorphous solids, the evaporation process can be affected by the glass transition temperature.
5. The material behaves as a solid below the glass transition temperature and as a liquid above it.
6. A liquid’s surface tension has an impact on the rate of evaporation. While a lower surface tension will enable the liquid to spread out and speed up evaporation, a higher surface tension will cause the liquid to form droplets and slow down the rate of evaporation.
7. The interactions between a solute and solvent as well as the intermolecular forces between the particles in a liquid cause these evaporation exceptions. They can affect how quickly water evaporates, but they cannot stop the process altogether.
EXPERIMENTS WITH EVAPORATION
Here are a few quick experiments that show how evaporation works:
1. Fill a container with water and leave it outside for it to evaporate. Watch the water level change over time. The water level will drop as the water evaporates, proving that water can transform into a gas through evaporation.
2. Fill a glass with cold water, then place a lid on top to prevent evaporation and condensation. Watch the glass as time passes. The water will condense and form droplets as it evaporates, showing how condensation works in reverse.
3. Fill two containers with the same amount of water and leave them exposed to the air to measure evaporation and surface area. The surface area of one container should be larger than the surface area of the other.
4.Watch the water levels develop over time. Evaporation is demonstrated to be faster with increased surface area by the water in the container with the larger surface area evaporating more quickly than the water in the container with the smaller surface area.
5. Fill two containers with the same amount of water and leave them exposed to the air to measure evaporation and temperature. Put one of the containers somewhere warm, and keep the other one somewhere cool. Watch the water levels develop over time. It will be clear that evaporation happens more quickly at higher temperatures because the water in the warm container will evaporate more quickly than the water in the cool container.
These experiments offer straightforward methods for viewing and comprehending the evaporation process. You can discover how variables like temperature, surface area, and air movement affect evaporation by observing and contrasting the outcomes of these experiments.
Do You Stack Up Against the Best?
If you have 30 minutes, try our free diagnostics test and assess your skills.
Evaporation FAQS
Evaporation: what is it?
A liquid changes from a liquid into a gas or vapour through the process of evaporation. When liquid particles accumulate enough energy to rupture their intermolecular bonds and transform into gases, this phenomenon takes place.
What elements influence evaporation?
Surface area, air pressure, temperature, and other variables all affect how quickly water evaporates. In general, evaporation happens more quickly at higher temperatures, lower air pressures, and more surface area and air movement.
What types of evaporation are there?
The evaporation of water from a puddle, the drying of sweat from the skin, and the drying of clothing on a clothesline are examples of evaporation.
What causes evaporation in the natural world?
The natural water cycle depends heavily on evaporation. It happens when the sun heats water from rivers, lakes, and oceans, turning it into water vapour. When this water vapour rises into the atmosphere, it has the potential to condense and form clouds before eventually returning to earth as precipitation.
Is reversing evaporation possible?
Yes, evaporation can be stopped by the condensation process, in which a gas or vapour turns back into a liquid.
What practical applications does evaporation have?
Many routine tasks rely on evaporation, including drying clothes, distilling water, and cooling through sweat evaporation. It is employed in industrial procedures like alcohol production and liquid purification.

Kathleen Currence is one of the founders of eTutorWorld. Previously a middle school principal in Kansas City School District, she has an MA in Education from the University of Dayton, Ohio. She is a prolific writer, and likes to explain Science topics in student-friendly language. LinkedIn Profile
Affordable Tutoring Now Starts at Just $22.49
eTutorWorld offers affordable one-on-one live tutoring over the web for Grades K-12. We are also a leading provider of Test Prep help for Standardized Tests (SCAT, CogAT, MAP, SSAT, SAT, ACT, ISEE, and AP).
What makes eTutorWorld stand apart are: flexibility in lesson scheduling, quality of hand-picked tutors, assignment of tutors based on academic counseling and diagnostic tests of each student, and our 100% money-back guarantee.
Whether you have never tried personalized online tutoring before or are looking for better tutors and flexibility at an affordable price point, schedule a FREE TRIAL Session with us today.
*There is no purchase obligation or credit card requirement
Grade 6 Science Worksheets
- Inquiry process
- Nature of Science
- Scientific Inquiry
- Inquiry, Analysis and Problem Solving
- Ethical Practices
- Science and Society
- Biotic and Abiotic Factors
- Impact of Organisms
- Adaptation
- Spheres of Earth
- Natural Resources
- Environmental Issues
- Conservation of Earth
- Understanding Technology
- Abilities To Do Technological Design
- Structure of Earth
- Solar System
- Rocks and Fossils
- Earth Systems
- Plate Tectonics
- Evolution
- Magnetic Field of Earth
- Geologic Time
- Materials and Processes That Shape a Planet
- Astronomy
- Ecology
- Energy
- Kinetic and Potential Energy
- Energy Transfer
- Matter and its Structure
- States of Matter
- Physical and Chemical Changes
- Force and Motion
- Electricity and Magnetism
- Wave Interactions
- Sound
- Light
- Introduction to Life Science
- The Origin & History of Life On Earth
- Plant and Animal Cells
- Parts of a Cell
- The Cell Cycle
- How Living Organisms Get Energy
- Classification of Organisms
- How Plants Grow & Reproduce
- The Human Respiratory System
- The Human Cardiovascular System
- The Human Digestive System
- The Human Endocrine Systems
- The Human Nervous System
- The Human Muscular System
- The Human Skeletal System
IN THE NEWS

Our mission is to provide high quality online tutoring services, using state of the art Internet technology, to school students worldwide.
Online test prep and practice
SCAT
CogAT
SSAT
ISEE
PSAT
SAT
ACT
AP Exam
Science Tutoring
Physics Tutoring
Chemistry Tutoring
Biology Tutoring
Math Tutoring
Pre-Algebra Tutoring
Algebra Tutoring
Pre Calculus Tutoring
Calculus Tutoring
Geometry Tutoring
Trigonometry Tutoring
Statistics Tutoring
Quick links
Free Worksheets
Fact sheet
Sales Partner Opportunities
Parents
Passive Fundraising
Virtual Fundraising
Our Expert Tutors
Safe and Secure Tutoring
Interactive Online Tutoring
After School Tutoring
Elementary School Tutoring
Middle School Tutoring
High School Tutoring
Home Work Help
Math Tutors New York City
Press
©2022 eTutorWorld Terms of use Privacy Policy Site by Little Red Bird
©2022 eTutorWorld
Terms of use
Privacy Policy
Site by Little Red Bird